Engineers are obsessed with reducing computing down to the size of one solitary chip these days. The smallest of transistors can operate at gigahertz far greater than computing speeds. The buzzword around engineering is 'nanoscale'. Everything is smaller and more efficient, engineers are always looking to minimize the amount of space something takes up. However, the smaller the object, the more 'nanoscale' some of the materials have to be to accommodate the size.
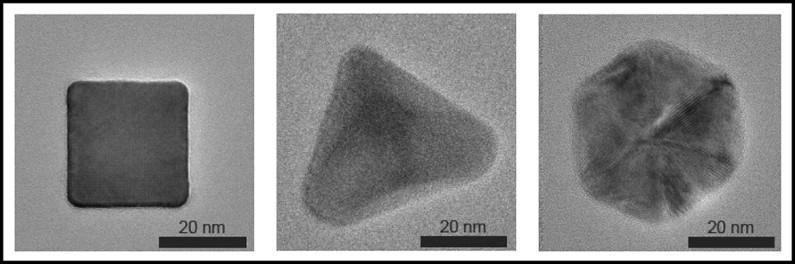 Researchers at Stanford University have investigated phase-changing nanoparticles to observe how their shape changes due to the arrangement of atoms within the crystals inside. They relate this to the charging and discharging of energy storage systems. This research would be valuable to any companies currently working with lithium-ion, hoping to release energy cells to the public.
Researchers at Stanford University have investigated phase-changing nanoparticles to observe how their shape changes due to the arrangement of atoms within the crystals inside. They relate this to the charging and discharging of energy storage systems. This research would be valuable to any companies currently working with lithium-ion, hoping to release energy cells to the public.
The work published in the Nature Materials journal was named Reconstructing solute-induced phase transformations within the individual nanocrystals.
Nanoparticles within energy storage systems enable the systems to charge faster and last longer. This process inspired the researchers to look into how deformed the crystals within the nanoparticles become during this process. They rationalize that if they can discover a breakthrough, they could improve charging and discharging and lifespan of batteries.
The abstract said:
Strain and defects can significiantly impact the performance of functional nanomaterials. This effect is well expemplified by energy storage systems, in which structural changes such as volume expansion and defect generation govern the phase transformations associated with charging and discharging.
The rational design of next-generation storage materials therefore depends cruciailly on understanding the correlation between the structure of individual nanoparticles and their solute uptake and release.
Jennifer Dione is an assistant professor of materials science and engineering at Stanford and worked on the project. They took lithium-ion batteries and tested the reactions of individual particles during charging. Using an environmental transmission electron microscope, they were able to observe the nanoparticles and at what hydrogen pressures they were reacting under. They found that the structure of a nanoparticle does have an effect on how an energy storage unit would perform. The difference is how hydrogen is absorbed in the center of a nanoparticle, which causes performance issues.
Tarun Narayan, a lead co-author of the study, told Stanford News: "This instrument is one of only a handful of its kind and allows us to study materials in their working environment.
Andrea Baldi, a postdoctoral co-author and faculty member of the Dutch Institute for Fundamental Energy Research (DIFFER) said: "Each technique offers different information that can be combined to gain a complete, multi-dimensional understanding of the system."
Robert Sinclair, a professor of materials science and engineering who also wrote alongside colleagues at Stanford said: "We could not have envisaged making in situ observations like this at the atomic level even a few year ago, and so what the teams had demonstrated and achieved is remarkable in the materials imaging field."
Dionne concluding by saying: "With this ability to peer inside nanoparticles during their operation, we can help design champion materials for next-generation energy storage devices."
